ICSE 10 Chemistry Competency Focused Practice Questions Solution:
IX. Long Answer Questions
Question 108. [Metallurgy]
With respect to the Hall Heroult process related to the extraction of aluminium, justify
the following:
(a) Powdered Coke is sprinkled over the electrolytic mixture undergoing electrolytic
reduction.
(b) Graphite anodes are continuously replaced during the electrolysis.
(c) Cryolite and fluorspar must be added to the electrolytic mixture.
Answer:
(a) Powdered Coke is sprinkled over the electrolytic mixture in electrolytic
reduction to avoid the carbon anodes from getting burnt during the electrolysis reaction.
It also helps to prevent heat loss by radiations from the molten electrolyte.
(b) Oxygen is liberated at the anode and reacts with carbon in the graphite anodes to form carbon dioxide, thus slowly burns away the anode; so, we have to replace the anode continuously.
(c) (1) Alumina has very high melting point (around 2000∘C). Cryolite and fluorspar lower the fusion temperature of the mixture containing alumina from 2000∘C to 1000∘C, thereby saving electrical energy.
(2) They enhance the conductivity and the mobility of the fused mixture.
Question 109. [Study of Compounds]
Write complete and balanced equations for the reactions occurring in the following cases:
(a) Passing dry ammonia gas over heated lead oxide placed in a combustion tube to
produce a silvery grey metal.
(b) When concentrated nitric acid is reacted with zinc to produce a reddish-brown gas.
(c) When concentrated sulphuric acid oxidises sulphur to produce a gas which turns
acidified potassium dichromate paper green.
Answer:
(a) NH3 + 3PbO → 3Pb + 2H2O + 2NO2
(b) Zn + 4HNO3 → Zn (NO3)2 + 2H2O + 2NO2
(c) 2H2SO4 + S → 3SO2 + 2H2O
Question 110. [Analytical Chemistry]
Give balanced equations for the conversions A, B and C.
A B C
Zn → ZnCl2 → Zn(OH)2 → ZnSO4
Answer:
(a) Zn +2HCl → ZnCl2 + 2HCl
(b) ZnCl2 + 2NaOH → Zn (OH)2 + 2NaOH
(c) Zn (OH)2 + H2SO4 → ZnSO4 + 2H2O
Question 111. [Electrolysis]
Rohan wants to electroplate a spoon with nickel.
(a) To which electrode should he connect the article to be electroplated?
(b) Write the equation for the reaction that will occur at the cathode.
(c) What should the anode be made up of?
Answer:
(a) Rohan should connect the spoon to cathode.
(b) Ni+2 + 2e– → Ni
(c) Anode should be a block of nickel.
Question 112. [Mole Concept and Stoichiometry]
Amit found that 30g of a gas occupied 1000 c.c at STP.
(a) What will the gram molecular weight and the vapour density of this gas be?
(b) How many molecules of this gas will be present in 44.8 l of it?
Answer:
(a) 1000 cc of the gas weighs 30g
So, 22400cc of the gas weighs = 30 x22400/1000 = 672g
Molecular weight of the gas = 672g
V.D of the gas = 672/2 = 336
(b) 22.4 litres of the gas contains 6.023×10²³ molecules
So, 44.8 litres of the gas contain 6.023×10²³ x 2 molecules
= 12.046 x 10²³ molecules
Question 113. [Study of Acids, Bases and Salts]
An element X combines with oxygen to form an oxide X2O3. This oxide is a good
conductor of electricity and can be reduced to its metal only by electrolysis.
(a) Write the equation for the reaction formed when the oxide (X2O3) combines with
hydrochloric acid.
(b) How many valence electrons are present in the outermost shell of X?
(c) Will element X undergo oxidation or reduction?
Answer:
(a) X2O3 + 6HCl → 2XCl3 + 3H2O
(b) 3. By the formula X2O3 we can see that valency of X is 3. Hence it is a metal having 3 valence electrons.
(c) Element X being a metal will undergo oxidation.
Question 114. [Analytical Chemistry]
Observe the reactions given below and answer the following questions:
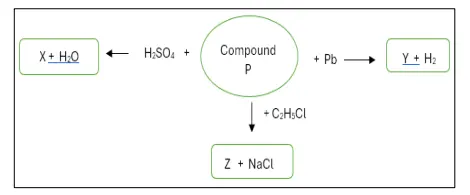
(a) Identify compound P.
(b) Give the chemical formula of Z.
(c) Write the reaction taking place between the identified compound P and sulphuric
acid.
(d) Name compound Y.
Answer:
(a) NaOH
(b) C2H5OH
(c) NaOH + H2SO4 → Na2SO4 + H2O
(d) Sodium plumbite
Question 115. [Periodic Properties and Variations of Properties]
Study the information given in the table below and answer the questions that follow.
(Note- the letters do not represent the actual symbols of the elements)
| Element | Electronic configuration | Ionisation energy (kJ mol-1) |
| X | 2, 2 | 900 |
| Y | 2, 8, 2 | 738 |
| Z | 2, 8, 8, 2 | 590 |
(a) Explain why element X has highest ionisation energy.
(b) To which period does Z belong?
(c) Draw the electron dot structure of the compound formed between Z and oxygen.
Answer:
(a) As the number of shells are less, the attraction by the nucleus on the electrons is
more so more energy is required to remove the electron from the outermost shell. Hence, Eeement X has highest ionisation energy.
(b) Z has 4 shells so, it belong to fourth period.
(c)
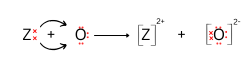
Question 116. [Study of Acids, Bases and Salts]
A student prepared a Potassium sulphite solution in the lab and added few drops of barium
nitrate solution to it. He observed a white precipitate being formed in the test tube. On
addition of dilute hydrochloric acid to the white precipitate and mixing it, he observed
that the precipitate disappeared.
(a) Name the white precipitate.
(b) Write a balanced chemical equation for the reaction between dilute hydrochloric acid
and the white precipitate.
(c) Name the gas evolved in the above reaction.
Answer:
(a) Barium sulphite. The equation of the reaction is as follows:
K2SO3 + Ba(NO3)2 → BaSO3 + KNO3.
(b) BaSO3 + 2HCl → BaCl2 + H2O + SO2
(c) The gas evolved is sulphur dioxide.
Question 117. [Mole Concept and Stoichiometry]
The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has a relative molecular
mass of 54. (At wt: H = 1, C = 12)
(a) What is the molecular formula of the hydrocarbon?
(b) Draw the structural formula of the hydrocarbon.
(c) Give the general formula of the hydrocarbon.
Answer:
(a) C4H6
(b)
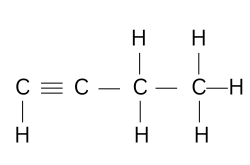
(c) CnH2n-2
Question 118. [Electrolysis]
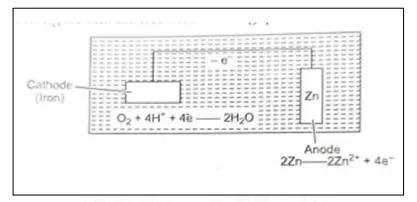
Study the given figure and answer the given questions:
(a) Identify the application of electrolysis demonstrated above.
(b) Which metal is protected in the above process?
(c) Why should the metal be protected?
Answer:
(a) Electroplating
(b) Iron
(c) The metal should be protected to prevent it from rusting.
Question 119. [Organic Chemistry]
Nita’s father bought a basket of ripe mangoes. While opening it she found a small sachet
containing a white crystalline powder along with the mangoes. She was told that it is a
chemical that releases a gas when it comes in contact with moisture, that induces ripening
of fruits.
(a) Name the chemical powder in the sachet.
(b) Name the gas.
(c) Give a balanced chemical equation for the reaction that results in the evolution of
this gas.
Answer:
(a) Calcium Carbide
(b) (ethyne/acetylene)
(c) CaC2 + 2H2O → Ca(OH)2+ C2H2
Question 120. [Periodic Properties and Variations of Properties]
Atomic number of element M is 12 and it forms an ionic compound with element L.
(a) Which of the following atomic numbers will match L?
i. 14
ii. 10
iii. 8
(b) What is the name given to the members of the group to which element M belongs?
(c) Draw the electron dot structure of the compound formed between M and L.
Answer:
(a) iii. 8.
(b) Alkaline earth metals. The electronic configuration of M is 2, 8, 2. So, it belongs to group 2.
Question 121. [Study of Compounds]
When two dry gases, oxygen and X, are passed over heated platinum, reddish-brown
fumes are seen in the receiving flask, as shown in the figure.
(a) Name the gas X.
(b) Give equation(s) for the reaction(s) that resulted in the formation of brown fumes.
Answer:
(a) NH3
(b)
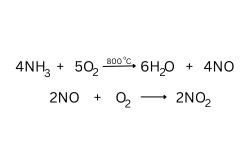
ICSE 10 Chemistry Competency Focused Questions Solution:
ICSE Related Links
Competency Focused Practice Questions
Chapter-wise Quiz/MCQ/Test:
ICSE Chapter wise Quiz For Class 6
ICSE Chapter wise Quiz For Class 7
ICSE Chapter wise Quiz For Class 8
ICSE Chapter wise Quiz For Class 9
ICSE Chapter wise Quiz For Class 10
Sample Papers
Board Papers
ICSE Class 9 Board Exam Papers
ICSE Class 10 Board Exam Papers
CBSE Related Links
Chapter wise Quiz/MCQ/Test
CBSE Chapter-wise Quiz for Class 6
CBSE Chapter-wise Quiz for Class 7
CBSE Chapter-wise Quiz for Class 8
CBSE Chapter-wise Quiz for Class 9
CBSE Chapter-wise Quiz for Class 10
Sample Papers
Board Papers
CBSE Class 10 Previous years’ Board Papers
Subscribe to our YouTube channel for more educational content.

