ICSE 10 Chemistry Competency Focused Practice Questions Solution
VIII. Short Answer Questions
Question 91. [Study of Acids, Bases and Salts]
Some general rules for the solubility of salts in water are listed.
- Carbonates are insoluble (except ammonium carbonate, potassium carbonate and
sodium carbonate). - Chlorides are soluble (except lead (II) chloride and silver chloride).
- Nitrates are soluble.
- Sulphates are soluble (except barium sulphate, calcium sulphate and lead (II)
sulphate).
Which substances produce an insoluble salt when aqueous solutions of them are mixed?
Justify your answer.
(a) Copper nitrate and magnesium chloride
(b) Zinc chloride and ammonium nitrate
(c) Silver nitrate and zinc chloride
(d) Potassium carbonate and sodium sulphate [Analysis]
Answer:
(c) silver nitrate and zinc chloride
Silver chloride is formed, which is an insoluble precipitate, while for all others, no
precipitate is formed
2AgNo3 + ZnCl2 → 2AgCl (s) + Zn(No3)2
Question 92. [Study of Compounds]
Ammonia gas is passed into water as shown below:
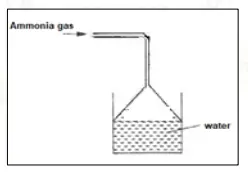
(a) When a red litmus paper was dropped into the resulting solution, it turned blue.
Which ions in the solution would have resulted for the colour change in the litmus
paper?
(b) Why is the funnel kept in an inverted position?
Answer:
(a) OH ions/ hydroxyl ions.
(b) To prevent back suction of water or to increase area of absorption.
Question 93. [Study of Compounds]
In the Haber’s process, the optimum yield of ammonia is obtained when a temperature
of 450oC -500oC, a pressure of 200 atmospheres, an iron catalyst and promoter
molybdenum are used.
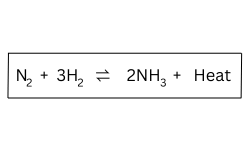
How and why would the yield of ammonia be affected if the temperature was raised to
600oC?
Answer:
As the forward reaction is exothermic, increasing the temperature will make the
reaction to reverse and thereby decrease the yield of ammonia.
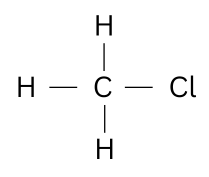
Question 94. [Organic Chemistry]
Give the structural formula and the name of the organic product formed when equal
volumes of methane and chlorine react together.
Answer:
The organic product formed is Chloromethane / methyl chloride.
CH4 + Cl2 → CH3Cl + HCl
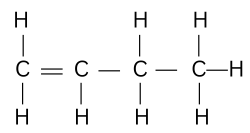
Question 95. [Organic Chemistry]
Complete combustion of one mole of a hydrocarbon produced four moles of carbon
dioxide and four moles of water only.
(a) Write the equation for the combustion reaction.
(b) Draw the structure of the hydrocarbon.
Answer:
(a) Complete combustion of one mole of a Butene produces four moles of carbon
dioxide and four moles of water. The equation of the reaction is as follows:
C4H8 + 6O2→ 4CO2 + 4H2O
(b)
Question 96. [Study of Compounds]
To the acid prepared by the contact process, Barium chloride solution is added. State
one observation and write an equation for the reaction that occurs.
Answer:
H2SO4 is prepared by Contact process. When H2SO4 reacts with Barium chloride solution, white ppt. of Barium sulphate is formed.
H2SO4 +BaCl2 → BaSO4 + 2HCl
Question 97. [Study of Compounds]
Platinum catalyst is used in the catalytic oxidation of ammonia.
(a) Write an equation for the reaction that occurs in the above case.
(b) Why does the platinum continue to glow even after the heating is discontinued?
Answer:
(a) The equation for the catalytic oxidation of Ammonia is as follows:
4NH3 + 5O2 → 4NO+ 6H2O
(b) The platinum continues to glow even after heating is discontinued because the
reaction is exothermic.
Question 98. [Study of Compounds]
With reference to the reaction occurring in the given figure: –
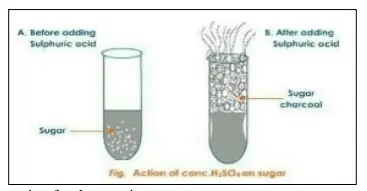
(a) Write an equation for the reaction.
(b) State the property of sulphuric acid exhibited in the above case.
Answer:
(a) The following is the reaction between concentrated sulphuric acid and sugar:
C12H22O11 + conc. H2SO4 + 12C + 11H2O
(b) Conc. H2SO4 is a dehydrating agent
Question 99. [Study of Compounds]
Brown ring test is used for the identification of nitrate ions.
(a) Why is freshly prepared Ferrous sulphate solution used in the above test?
(b) What is the chemical name of the brown ring?
Answer:
(a) If Ferrous sulphate is left exposed to air for some time, then it will be oxidised to
ferric sulphate and it will not respond to the test.
(b) Chemical name of the brown ring is Nitroso ferrous sulphate.
Question 100. [Mole Concept and Stoichiometry]
Ravi heated 367.5 g of KClO3 in a test tube. The decomposition of potassium chlorate
took place according to the equation.
2KClO3 → 2KCl + 3O2
Find:
(a) the volume of the colourless and odourless gas liberated during the experiment.
(b) the weight of the residue left behind in the test tube.
Answer:
(a) Molecular mass of KClO3 = 39 + 35.5 +16 x 3 = 39 + 35.5 + 48= 122.5
2KClO3 = 2 x 122.5 = 245 g
O2 occupies 22.4 lt of space.
Therefore, space occupied by 3O2 = 3 x 22.4 = 67.2 lt.
Now, 245g of KClO3 liberates 67.2 l of O2
So, 367.5g of KClO3 gives = 67.2 x 367.5/245= 100.8 lt of O2
Hence, volume of O2 liberated during the experiment is 100.8 lt.
(b) Molecular mass of KCl = 39 + 35.5 = 74.5 g
2×122.5g of KClO3 forms 74.5g of KCl
So, 367.5g of KClO3 forms = 74.5 x 367.5/245=111.75 g of KCl
Hence, weight of the residue left behind in the test tube is 111.75 g.
Question 101. [Metallurgy]
For construction work the alloy of Aluminium i.e. Duralumin is used rather than pure
Aluminium. Give two valid reasons.
Answer:
The two reasons are:
(i) Duralumin is light and strong, while aluminium is light and weak. It is also unaffected
by moist air.
(ii) It is corrosion-resistant and has high tensile strength.
Question 102. [Mole Concept and Stoichiometry]
Ram took 5 moles of carbon atoms in a container, and Krish took 5 moles of sodium
atoms in another container of the same volume.
(a) Whose container is heavier?
(b) Which container has a larger number of atoms?
Answer:
(a) Atomic mass of carbon is 6u and atomic mass of sodium is 11u. Mass of 5 moles of carbon atoms= 5 x 6= 30g and mass of 5 moles of sodium= 5 x 11 = 55g. Hence, Krish’s container is heavier.
(b) Both the containers have the same number of atoms as they contain same number of moles.
Question 103. [Analytical Chemistry]
A, B and C are three elements which undergo chemical reactions according to the
following equations:
A2O3 +2B → B2O3 + 2A
3CSO4 + 2B→ B2(SO4)3 +3C
3CO + 2A → A2O+3C
Answer the following questions:
(a) Which element is the most reactive?
(b) Which element is the least reactive?
Answer:
(a) Since B displaces A in 1st reaction and C in 2nd reaction. Therefore, it is positioned higher than A and C in reactivity series. Hence, B is the most reactive element.
(b) Since C gets displaced by both A and B, hence, C is the least reactive element.
Question 104. [Electrolysis]
PQ2 is a hard crystalline solid having high melting and boiling points. It is a good
conductor of electricity in both molten and aqueous forms.
(a) The conductivity of PQ2 is due to the presence of free ________ .(ions, molecules,
electrons)
(b) During electrolysis of aqueous PQ2, if thickening of the cathode and thinning of the
anode is observed, the anode material will be _________ . (graphite, metal P)
Answer:
(a) Ions
(b) Metal P
Question 105. [Metallurgy]
A student was asked to draw the flowchart for the extraction of zinc from zinc blende
based on the principles of Metallurgy. What he drew is given below.
2 steps out of the 5 were incorrect. Identify and correct them.

Answer:
Since it is concentration of sulphide ore of zinc, the incorrect steps are:
(a) Step 2: It should be froth floatation instead of leaching
(b) Step 3: It should be roasting instead of calcination.
Question 106. [Organic Chemistry]
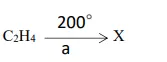
Given above is the representation of the conversion of ethene to a saturated hydrocarbon
X, where ‘a’ stands for the catalyst.
(a) Identify ‘a’.
(b) Give the complete chemical equation for the conversion of C2H4 to X.
Answer:
(a) Nickel
(b)
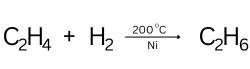
Question 107. [Organic Chemistry]
The description of an organic compound is as follows:
(a) Molecular formula is C3H8O.
(b) Functional group is attached to the first carbon atom.
(c) Reacts with sodium at room temperature with brisk effervescence, releasing
hydrogen gas.
Identify the compound and draw its structure.
Answer:
The compound is Propanol. Its structure is as follows:
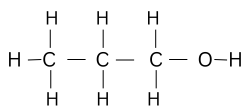
ICSE 10 Chemistry Competency Focused Questions Solution:
ICSE Related Links
Competency Focused Practice Questions
Chapter-wise Quiz/MCQ/Test:
ICSE Chapter wise Quiz For Class 6
ICSE Chapter wise Quiz For Class 7
ICSE Chapter wise Quiz For Class 8
ICSE Chapter wise Quiz For Class 9
ICSE Chapter wise Quiz For Class 10
Sample Papers
Board Papers
ICSE Class 9 Board Exam Papers
ICSE Class 10 Board Exam Papers
CBSE Related Links
Chapter wise Quiz/MCQ/Test
CBSE Chapter-wise Quiz for Class 6
CBSE Chapter-wise Quiz for Class 7
CBSE Chapter-wise Quiz for Class 8
CBSE Chapter-wise Quiz for Class 9
CBSE Chapter-wise Quiz for Class 10
Sample Papers
Board Papers
CBSE Class 10 Previous years’ Board Papers
Subscribe to our YouTube channel for more educational content.

