ICSE Class 10 Chemistry Competency Focused Practice Questions Solution:
V. Structural diagram: (1 Mark each)
Question 69. [Organic Chemistry]
Draw the structural diagram of the product obtained when ethene reacts with chlorine.
Answer:
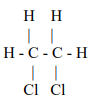
Explanation: When ethene reacts with chlorine, the product formed is 1,2-dichloro ethane.
CH2 = CH2 + Cl2 → ClCH2 – CH2Cl
Question 70. [Organic Chemistry]
An organic acid on cooling below 16.5°C crystallises out in the pure form, forming a
crystalline mass resembling ice. Draw the structural diagram of this carboxylic acid.
Answer:
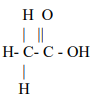
Explanation: Acetic acid or ethanoic acid on cooling below 16.5°C crystallises out in the pure form, forming a crystalline mass resembling ice. Therefore, it is also called glacial acetic acid.
Question 71. [Chemical Bonding]
Magnesium ribbon is added to dilute HCl. A gas is liberated along with the formation of
a compound. Draw an electron dot diagram to show the structure of the compound that
is formed.
Answer:
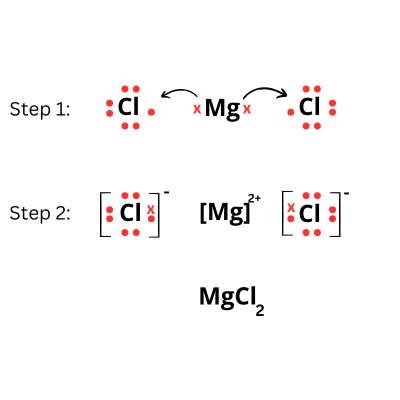
Explanation: The equation is as follows:
Mg + HCl → MgCl2 + H2
As MgCl2 is an electrovalent compound, we will show the electron dot structure, where Magnesium donates two electrons and each chlorine accepts one electron to become stable.
Question 72. [Organic Chemistry]
Draw the chain Isomer for the following organic compound.
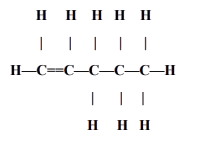
Answer:
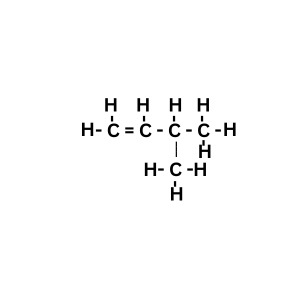
Explanation: The structural diagram given in the question is of pentene. Its chain isomer is 3 methyl but-1-ene.
Question 73. [Chemical Bonding]
Calcium hydroxide dissolves in water and forms a positive ion and a negative ion. Draw
the structure of the negative ion.
Answer:
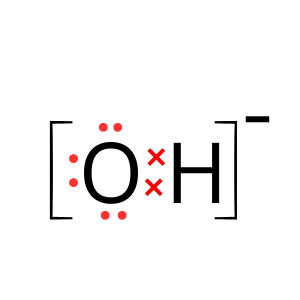
Explanation: Calcium hydroxide contains Ca2+ and OH– ions: this is two positive charges and one negative charge. Hence, we will have to draw the structure of hydroxyl ion.
Question 74. [Organic Chemistry]
Draw the structure of the following organic compounds:
2 – methyl butane
Answer:
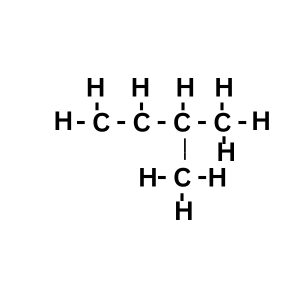
Explanation: 2 – methyl butane will have 4 carbon atoms in a row and one carbon atom will be bonded with second carbon atom to make methyl.
Question 75. [Chemical Bonding]
The equation given above represents the molecule formation of element X. Fill in the
box with the electron dot structure of the molecule.
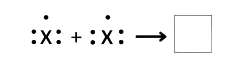
Answer:
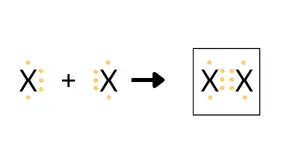
Explanation: Atom X in the given question has 5 electrons in its valence shell. To attain stability, it needs 3 more electrons. Hence, both the atoms of X will make a triple covalent bond.
Question 76. [Chemical Bonding]
Draw an isomer of the given structure:

Answer:
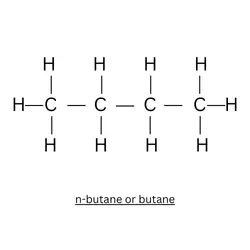
Explanation: The structure given in the question is 2 – methyl propane. This is an isomer of butane. Butane has 2 isomers: n-butane and iso-butane (2 – methyl propane).
ICSE Class 10 Chemistry Competency Focused Questions Solution:
ICSE Related Links
Competency Focused Practice Questions
Chapter-wise Quiz/MCQ/Test:
ICSE Chapter wise Quiz For Class 6
ICSE Chapter wise Quiz For Class 7
ICSE Chapter wise Quiz For Class 8
ICSE Chapter wise Quiz For Class 9
ICSE Chapter wise Quiz For Class 10
Sample Papers
Board Papers
ICSE Class 9 Board Exam Papers
ICSE Class 10 Board Exam Papers
CBSE Related Links
Chapter wise Quiz/MCQ/Test
CBSE Chapter-wise Quiz for Class 6
CBSE Chapter-wise Quiz for Class 7
CBSE Chapter-wise Quiz for Class 8
CBSE Chapter-wise Quiz for Class 9
CBSE Chapter-wise Quiz for Class 10
Sample Papers
Board Papers
CBSE Class 10 Previous years’ Board Papers
Subscribe to our YouTube channel for more educational content.

