ICSE Class 10 Chemistry Competency Focused Practice Questions Solution
IV. One Word Answer: (1 Mark each)
Question 59. [Analytical Chemistry]
Name a positive non-metallic radical which is basic in nature.
Answer: Ammonium (NH4+)
Explanation: In ammonia the two electrons remaining on the nitrogen atom form a bond with the hydrogen ion. This bond that is formed with the fourth hydrogen is known as a coordinate (or dative covalent) bond because nitrogen provides both of the electrons. Since nitrogen donates both the electrons, the ammonium ion attains a positive charge.
Question 60. [Organic Chemistry]
How many electrons are present in one molecule of CH4?
Answer: 10
Explanation: Atomic no. of C is 6 and of H is 1. Hence total electrons = 6 + (4 x 1) = 10
Question 61. [Organic Chemistry]
Identify the longest carbon chain and mention the number of carbons present in it.
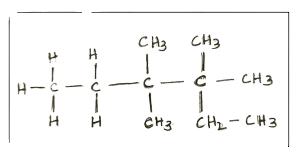
Answer: 6
Explanation:
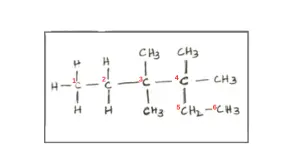
Question 62. [Mole Concept and Stoichiometry]
Gas M occupies a volume of 1000 c.c and contains X molecules. How many molecules
will be present in gas N occupying a volume of 250 c.c?
Answer: X/4
Explanation: Avogadro’s law states that equal volumes of different gases contain an equal number of molecules under the same conditions of temperature and pressure. As gas N is occupying 1/4th the volume of gas M (1250 / 1000 = 1/4), therefore gas N has 1/4th molecules as compared to gas M.
Question 63. [Periodic Properties and Variations of Properties]
Element X belongs to period 2 and group 1 of the periodic table. State the formula of
the chloride of the element X.
Answer: XCl
Explanation: As element X belongs to period 2 and group 1 of the periodic table, therefore its electronic configuration is 2, 1. Its valency is 1+ . Valency of Cl is 1– . Hence molecular formula will be XCl.
Question 64. [Study of Compounds]
Anurag added dilute H2SO4 to a given sample X and heated the mixture. He observed
that a gas was liberated which had a foul smell of rotten eggs and it turned moist lead
acetate paper silvery black. Name the gas evolved in the above case.
Answer: Hydrogen sulphide gas
Explanation: When a dilute acid is reacted with a metal sulphide, its salt and hydrogen sulphide gas are formed which has a smell like rotten egg.
For example: H2SO4 + ZnS → ZnSO4 + H2S
Question 65. [Organic Chemistry]
Name the alkyl component of acetic acid.
Answer: -CH3 (methyl)
Explanation: Formula of acetic acid is CH3COOH. In this formula, alkyl component is CH3, which is methyl.
Question 66. [Mole Concept and Stoichiometry]
28g of nitrogen and 44g of carbon dioxide at the same conditions of temperature and
pressure occupy the same amount of space. What term describes such space occupied
by any gas?
Answer: Molar volume
Explanation: Space occupied by molecular mass of a substance is called molar volume. Molecular mass of carbon dioxide gas is 44g and that of nitrogen gas is 28g.
Question 67. [Study of Compounds]
When copper reacts with a hot dilute solution, reddish-brown fumes are observed.
Another compound, P, having the same anion that is present in the hot solution on
heating, melts into a colourless liquid, releasing only oxygen gas without any coloured
fumes. Identify P.
Answer: Sodium nitrate / Potassium nitrate
Explanation: Copper reacts with hot dilute nitric acid forming copper nitrate and nitrogen dioxide (reddish-brown gas) and water.
Cu (s) + 4HNO3(aq) ⇢ Cu(NO3)2 (aq) + 2NO2(g) + 2H2O(aq)
When Sodium nitrate or Potassium nitrate is heated, it decomposes into a colourless liquid called sodium/potassium nitrite and oxygen gas.
NaNO3 ⇢ NaNO2 + O2
KNO3 ⇢ KNO2 + O2
Question 68. [Metallurgy]
Calcite (CaCO3), a sedimentary rock, is found most abundantly in many geological
environments. It has a perfect cleavage in 3 directions, which makes it the most difficult
rock to cut, and moreover, the labour of cutting calcite is also very high. What term
related to metallurgy will suitably describe Calcite in the context of extracting calcium
from calcite?
Answer: Mineral
Explanation: Mineral is a naturally occurring inorganic solid with a definite chemical composition and a crystalline structure.
ICSE Class 10 Chemistry Competency Focused Questions Solution:
ICSE Related Links
Competency Focused Practice Questions
Chapter-wise Quiz/MCQ/Test:
ICSE Chapter wise Quiz For Class 6
ICSE Chapter wise Quiz For Class 7
ICSE Chapter wise Quiz For Class 8
ICSE Chapter wise Quiz For Class 9
ICSE Chapter wise Quiz For Class 10
Sample Papers
Board Papers
ICSE Class 9 Board Exam Papers
ICSE Class 10 Board Exam Papers
CBSE Related Links
Chapter wise Quiz/MCQ/Test
CBSE Chapter-wise Quiz for Class 6
CBSE Chapter-wise Quiz for Class 7
CBSE Chapter-wise Quiz for Class 8
CBSE Chapter-wise Quiz for Class 9
CBSE Chapter-wise Quiz for Class 10
Sample Papers
Board Papers
CBSE Class 10 Previous years’ Board Papers
Subscribe to our YouTube channel for more educational content.

